Animal medicines and the environment
There is increasing interest in protecting the environment from avoidable risks. This includes awareness that medicine residues may enter the environment, with potentially unwanted effects.
The most common route for pharmaceuticals into the environment is through excretion- by both people and animals – after they have been taken. From there, they can find their way into soil or water.
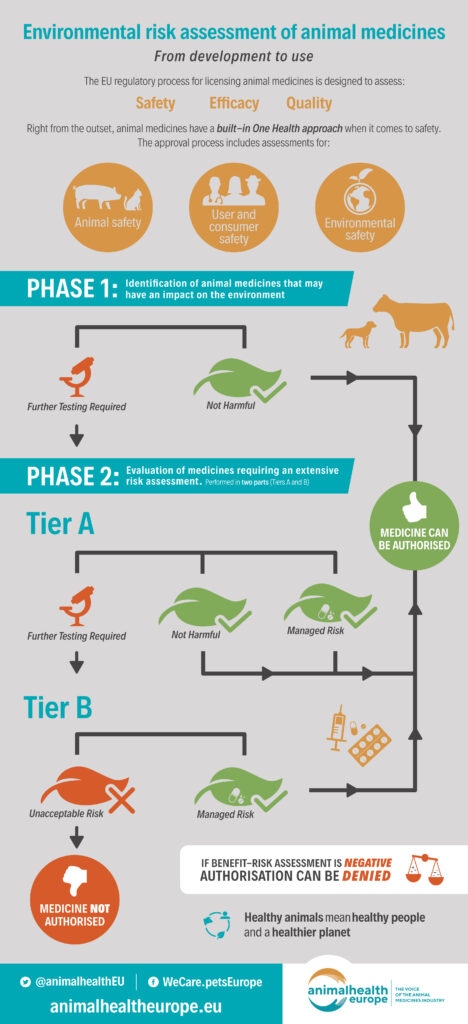
In the case of animal medicines, any application for marketing must include an Environmental Risk Assessment (ERA) that details any potential risk posed to the environment. With this in mind, the use of medicines in agriculture is strictly controlled to minimise the levels of pharmaceutical residues that reach the environment. And any animal medicines not meeting with the requirements of the environmental risk assessment are denied authorisation for use.
An independent and robust evaluation and authorisation process for animal medicines means that, before reaching the market, their quality, safety, and efficacy is assessed as part of a benefit-risk evaluation. Right from the outset, animal medicines have a built-in One Health approach as they are tested for animal, user and consumer, and environmental safety.
AnimalhealthEurope’s members comply with all relevant independent regulatory processes for authorising medicines. Used appropriately and in accordance with the manufacturers’ label and instructions, animal medicines do not pose a major risk to the environment. Protecting animals, people and the environment is and will remain a priority for our industry.
Infographics